

|
© Copyright 2004-2021 PharmaInformatic Boomgaarden. All rights reserved. Site map Contact Terms of Use Imprint |
PharmaInformatic provides
pharmacokinetic
ADME Knowledge Bases
and AI-based
Expert Systems
for drug discovery
and toxicological
risk assessment.

Project results
of our research project
EXITOX-II
(Animal-free toxicity testing)
were presented at the
BMBF-Statusseminar.

The company was founded in 2004 and is based in Emden, Germany.
Get In Contact
![]() LinkedIn
LinkedIn ![]() Twitter
Twitter
Replacement of animal tests by Artificial Intelligence
|
Once a suitable drug candidate is found, it needs to be checked if the drug is taken up and effective in humans. Our technology (expert systems) evaluates the pharmacokinetic profile of drugs in humans prior to clinical trials: |
||
|
|
||
|
New expert system to evaluate Plasma Protein Binding by Artificial Intelligence: |
||
|
|
PharmaInformatic has developed the largest and comprehensive annotated knowledge base on Plasma Protein Binding of compounds worldwide. It now contains more than 21.000 data records taken from more than 2.800 scientific publications. A validation study showed a high quality of prediction (more). The expert system is now available for drug research projects and toxicological risk assessment. Contact us for further details (email). |
|
|
IMPACT-F: Prediction of oral bioavailability in humans (drug-uptake) |
||
|
|
The expert system IMPACT-F evaluates oral bioavailability of future drugs in humans. The technology is used to optimise lead candidates. Predictions were shown to be much more precise than animal trials: they were as accurate as the common deviation between individual humans taking part in the same clinical trial. (research results). |
|
|
|
||
29.11.2021:
Montag 13-14 Uhr
Herbert-Stiller
Preisverleihung
in Köln
Link:
YouTube
Live Streaming

Research prize awarded
to
Dr. Wolfgang Boomgaarden, PharmaInformatic
for innovative,
human-relevant,
and animal-free
research
Further information
in English or German
Review article
on
oral bioavailability
and
regulatory aspects
published in
Pharmaceutics 2021
Free full text (open access)
Ersatz von Tierversuchen
durch Künstliche Intelligenz
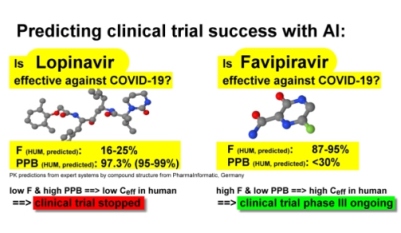 The evaluation with AI-based expert systems of two drugs for the treatment of COVID-19 showed that the effective concentration of Lopinavir would be too low to be effective in humans due to low oral bioavailability F and high protein binding PPB.
The evaluation with AI-based expert systems of two drugs for the treatment of COVID-19 showed that the effective concentration of Lopinavir would be too low to be effective in humans due to low oral bioavailability F and high protein binding PPB.
