Oral bioavailability of blockbuster drugs in humans and animals
Drug-uptake studies in animals, such as rats, dogs, monkeys and mice are still used to estimate oral bioavailability in humans. If oral bioavailability in animals is too low, for example less than 20%, a compound would be most often excluded for consideration as a development candidate.
But, drug-uptake studies in animals are often misleading: A comparison of oral bioavailability results from animals and humans for a large number of approved and established drugs in PACT-F showed, that oral bioavailability in animals is inconsistent with the values reported for humans and large differences can exist. This also applies to very successful commercial drugs, called blockbuster drugs, with annual sales of more than one billion US Dollar.
Aripiprazole and Esomeprazole, the most sold drugs of 2013 in the US market, have low oral bioavailability in animals, but drug-uptake in humans is high. From a commercial point of view, it is not advisable to carry out animals studies on oral bioavailability and trust in their results.
|
ARIPIPRAZOLE
Aripriprazole is an antipsychotic and antidepressant drug which is used in the treatment of schizophrenia, bipolar disorder and depression. It was first approved by the FDA in 2002. In 2013 it was the most sold drug in the U.S. market and achieved a total sales of US$ 6294 million. Large species differences in oral bioavailability were found between humans and animals. The following figure shows the differences of oral bioavailability in humans, rats, dogs, monkeys and mice:
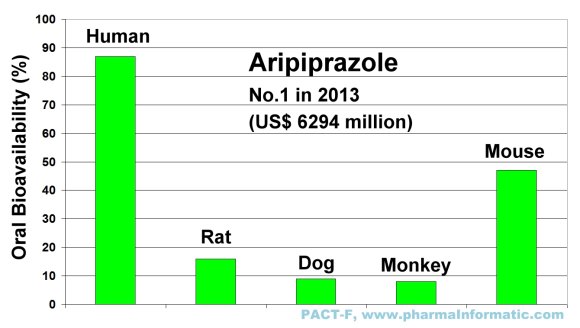 |
The blockbuster drug Aripriprazole has a very low oral bioavailability in rats, dogs and monkeys. If the developers of Aripriprazole had relied solely on drug-uptake results in rats or dogs or monkeys, the most sold blockbuster drug Aripriprazole with sales of US$ 6294 million in 2013 would be not available on the market today.
References:
Bauman, J.N. et al. Comparison of the bioactivation potential of the antidepressant and hepatotoxin nefazodone with aripiprazole, a structural analog and marketed drug. Drug Metab Dispos. 36, 1016-1029 (2008)
Swainston Harrison, T., Perry, C.M. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 64, 1715-1736 (2004)
European Medicines Agency: EMA/737723/2013 - Committee for Medicinal Products for Human Use (CHMP), Assessment report ABILIFY MAINTENA, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002755/WC500156105.pdf, May 2016
OMEPRAZOLE (ESOMEPRAZOLE)
Omeprazole and Esomeprazol are proton pump inhibitors, which reduce gastric acid secretion. Both drugs are used for the treatment of duodenal ulcer disease, erosive esophagitis and gastroesophageal reflux disease.
Omeprazole and Esomeprazol have achieved enormous amount of sales. Omeprazole was the first proton pump inhibitor on the market (brand name: Prilosec, AstraZeneca). It was approved in 1989 by the FDA. Esomeprazol, the S-enantiomer of Omeprazole, was approved by the FDA in 2001 (brand name: Nexium, AstraZeneca).
In 2010 and 2012 it was the most sold drug in the U.S. market. It achieved sales of US$ 5276 million in 2010 and sales of US$ 5639 million in 2012. In 2013 it was on rank two of the most sold drugs in the U.S. market (sales of US$ 5975 million).
The following figure shows the differences of oral bioavailability in rats, dogs and mice and humans of Omeprazole:
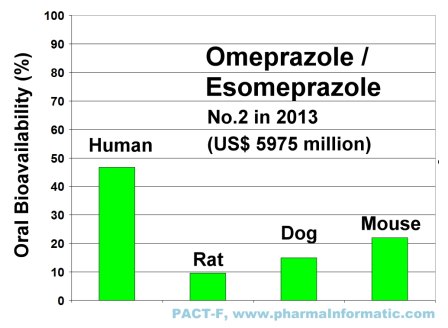 |
Drug-uptake of Omeprazol was high in humans, but low in rats, dogs and mice. Drug-uptake studies in animals often misguide drug discovery and development. The expert system IMPACT-F evaluates drug-uptake in humans much more reliable than animal trials and it helps to find the optimum oral dose in humans prior to first clinical trials.
The oral bioavailability of Esomeprazol, the S-enantiomer of Omeprazole, was investigated two clinical studies and oral bioavailability was found to be between 50-89% in 16 healthy human subjects (for details see here).
References:
Cederberg, C., Andersson, T., Skånberg, I. Omeprazole: pharmacokinetics and metabolism in man. Scand J Gastroenterol Suppl. 166, 33-40; discussion 41-42. Review. (1989)
Hassan-Alin, M., Andersson, T., Bredberg, E., Röhss, K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 56, 665-670 (2000)
Larsson, H. et al. Inhibition of gastric acid secretion by omeprazole in the dog and rat. Gastroenterology. 85, 900-907 (1983)
Uno, T. et al. Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol. 63, 143-149 (2007)
Regårdh, C.G., Andersson, T., Lagerström, P.O., Lundborg, P., Skånberg, I. The pharmacokinetics of omeprazole in humans--a study of single intravenous and oral doses. Ther Drug Monit. 12, 163-172 (1990)
Regårdh, C.G., Gabrielsson, M., Hoffman, K.J., Löfberg, I., Skånberg, I. Pharmacokinetics and metabolism of omeprazole in animals and man--an overview. Scand J Gastroenterol Suppl. 108, 79-94 (1985)
Tallman, M.N., Ali, S.Y., Smith, P.C. Altered pharmacokinetics of omeprazole in cystic fibrosis knockout mice relative to wild-type mice. Drug Metab Dispos. 32, 902-905 (2004)
Watanabe, K., Furuno, K., Eto, K., Oishi, R., Gomita, Y. First-pass metabolism of omeprazole in rats. J Pharm Sci. 83, 1131-1134 (1994)
DULOXETINE
Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor. It was first approved in 2004 by the FDA. In 2011 it was on rank nine of the most selling drugs in the U.S. market and achieved a total sales of US$ 3552 million.
In 2012 and 2013 the blockbuster Duloxetine was on rank five of the most selling drugs in the U.S. market and achieved sales of US$ 4473 million and US$ 5083 million, respectively.
An analysis of all records in PACT-F about duloxetine showed large species differences in oral bioavailability between humans, rats and dogs:
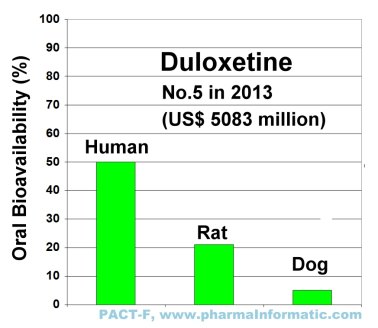 |
References:
Mudaliar, M. Bioequivalence comparison of generic drug DuLocap and brand drug Cymbalta. J Med Investig Pract 9, 79-84 (2014)
European Medicines Agency: Duloxetine, Scientific discussion, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000552/WC500026745.pdf, May 2016
Ethical and financial reasons speak against the use of drug-uptake experiments in animals. Alternative computational methods, such as the expert system IMPACT-F are now available and replace animal trials on drug-uptake. Pharmaceutical companies have applied the expert system to evaluate human oral bioavailability in various therapeutic areas such as diabetes, inflammation, antivirals, autoimmune diseases and cancer.
The expert system evaluates oral bioavailability in humans significantly more reliably than animal trials. This further increases the prospects of human clinical trials, because optimum oral dose for first-in-human clinical trials can be determined much more accurately. Efficacy issues are identified as the main reason why clinical trials in humans fail.
Animal trials can hamper drug discovery and development: Top selling drugs, including the most sold blockbuster drugs of 2013, would not have been developed if scientists had relied solely on bioavailability trials in animals.
Furthermore it raises the question, which other blockbuster drugs would be on the market today, if animal trials would have not been used to preselect compounds and drug-candidates for further development?
An evaluation with the expert system identifies, if back-up compounds or drug-candidates, which previously were rejected because of low animal bioavailability, would possess oral bioavailability in humans and could be further developed to oral medications.
Contact:
Dr. Wolfgang Boomgaarden
Founder and CEO of PharmaInformatic
(email)
We will continuously publish novel research findings and results on this website.
Follow us on Twitter or connect via LinkedIn:


Blockbusters
Drugs
are
Top Selling Drugs having sales
in excess of
US-$ one billion
in a year
such as
ARIPIPRAZOLE
No.1 in 2013
US$ 6294 million
ESOMEPRAZOLE
No.2 in 2013
US$ 5975 million
DULOXETINE
No.5 in 2013
US$ 5083 million
Source:
Drugs.com Statistics, U.S. Pharmaceutical Sales - 2013 (Link).


|
© Copyright 2004-2021 PharmaInformatic Boomgaarden. All rights reserved. Site map Contact Terms of Use Imprint |
 |
|
|
|
|
|
|
Bioavailability prediction of drug candidates for
treatment of Type 2 diabetes
(more)
“...and in short time our development project was taken to the next step."
 |
|
|
|
|
Recent News:
Selection and prioritisation of lead compounds for the treatment of inflammatory and autoimmune diseases based on estimated oral bioavailability in humans with IMPACT-F.
(more)
Novel models to forecast
Plasma Protein Binding
of compounds
show a high quality of prediction.
Based on a large
independent validation dataset.
a low error
and high correlation
between experimental and predicted PPB values was found.
 |
Selection and prioritisation
of prodrugs
for the treatment of
cancer
based on estimated oral bioavailability in humans
with IMPACT-F.
(more)
