|
IMPACT-F
|
|
Novel research findings on bioavailability of drugs
|
|
In drug development today, drug-uptake studies in animals are used as a guidance to decide if a compound would have sufficient oral bioavailability in humans. But, are these animal trials useful? Do they help to project drug-uptake in human?
Several articles have described species differences in oral bioavailability of a particular drug (click here for some references on Medline). Other research papers, such as from Sietsema (1989), Mahmood (2000), Chiou and Buehler (2002), Ward, Nagilla and Jolivette (2005), Cao et al. (2006), Hosea et al. (2009), Takahashi et al. (2009), Akabane et al. (2010) and other research groups have analysed species differences in oral bioavailability based on a larger number of drugs.
Due to the development of a large and comprehensively annotated collection of results from trials in animals and humans (PACT-F), it is now possible to provide a reliable answer to this fundamental question of life based on a large number of investigated drugs. The evaluation, provided here is based on more than hundred approved and marketed drugs, including top selling blockbuster drugs.
Furthermore, the knowledge base enables scientists to develop computational models to predict oral bioavailability in humans. Are they better or worse than animal models? Here are the results from our research:
- Overview: Quality of models to predict bioavailability
- Validation of the expert system
- Variability of bioavailability in humans (inter-subject variability)
- Species differences in oral bioavailability (the Ciprofloxacin-example)
- Update: Oral bioavailability of blockbuster drugs in humans and animals
- Conclusions
|
|
REPLACEMENT OF ANIMAL TESTING with Artificial Intelligence!
|
|
|
|
Overview: Quality of models to predict oral bioavailability in humans
|
|
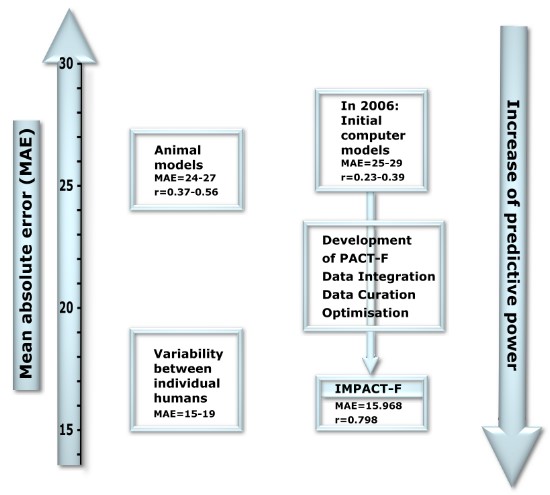
The knowledge base PACT-F contains a large number of bioavailability results from clinical trials in humans and from preclinical animal trials. We have calculated the quality (reliability) of animal models and of computational models to predict human oral bioavailability.
These results were compared with the inter-subject variability in humans, which was calculated by averaging the differences in bioavailability between individual humans taking part in the same clinical trial. This yielded to the following results:
|
|
|
|
The analysis showed that oral bioavailability in animals differed substantially compared to the measurements in humans. The mean absolute error (MAE=24-27) was high and only a low to medium correlation (r=0.37-0.56) was obtained between human and animal bioavailability values (comparison was based on more than 100 drugs).
Initial computational models, developed in 2006, were based on a low number of drugs and had low predictive power (MAE=25-29, r=0.23-0.39). Therefore we increased the number of drugs and their bioavailability results to learn from and developed the knowledge base PACT-F.
The development of PACT-F took many years: An enormous amount of clinical and preclinical data had to be integrated and compared. Chemical structures were painted, transformed and integrated into the knowledge base. Several errors in scientific publications were identified by data curation (due to the high degree of annotation) and were excluded from the knowledge base. The reliability of computer models, validated with large, diverse and independent test sets, increased continuously with size and quality of the knowledge base.
After eight years of development, we finally selected the best computer-models and integrated them into IMPACT-F. A final validation showed that the expert system calculates human oral bioavailability much more precisely than animal trials: A low error (MAE=15.968) and a good correlation (r=0.798) between experimental and predicted oral bioavailability values was observed.
The quality of prediction we obtained was high. Predictions on oral bioavailability were as accurate as the common deviation between individual humans taking part in the same clinical trial (inter-subject variability).
|
|
Validation of the expert system IMPACT-F
|
|
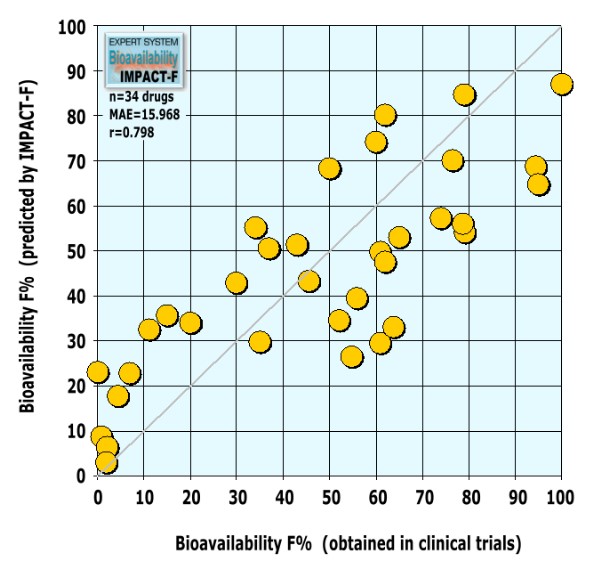
After the final expert system was completed, a literature search was conducted to find novel investigational drugs and their human bioavailability results in order to validate IMPACT-F. We found 34 novel drugs or drug candidates coming from different therapeutic areas such as cancer, antibacterial or antiviral infection, CNS, inflammation, gastrointestinal and renal diseases, metabolism related diseases and cardiovascular diseases. All new drugs were used to validate IMPACT-F. A human oral bioavailability prediction with IMPACT-F gave the following results:
|
|
|
|
Results of the evaluation based on thirty-four external drugs:
A low mean absolute error (MAE=15.968) and a good correlation (r=0.798) between experimental and predicted oral bioavailability values was observed. IMPACT-F calculates oral bioavailability in humans much more precisely compared to animal trials.
The expert system can forecast oral bioavailability of novel drugs reliably. Bioavailability predictions of IMPACT-F were as accurate as the common deviation between individual humans taking part in the same clinical trial (inter-subject variability).
Interestingly, the investigated 34 drugs possessed only a low structural similarity to all molecules in PACT-F: More than 82% of the drugs showed a maximal Tanimoto similarity below 0.67. This shows that highly similar drugs are not needed to estimate reliably oral bioavailability of entirely novel drug structures.
IMPACT-F uses and combines all available information in PACT-F from previous known bioavailability to create a reliable estimate of oral bioavailability in humans.
The expert system can predict oral bioavailability of small-molecule drug-candidates ranging across all therapeutic areas.
The validation set included a CCR5 antagonist for treatment of rheumatoid arthritis, a p38 MAP-kinase inhibitor for treatment of acute coronary syndrome, four novel angiotensin II receptor blocker (antihypertensive), a beta3-adrenoceptor agonist in development for treatment of overactive bladder, two novel HIV-1 non-nucleoside reverse transcriptase inhibitors (antivirals), a dual orexin receptor antagonist, three different cardiovascular drugs (treatment of atrial fibrillation, neutrophil elastase inhibitors), four new cancer drugs (an ALK and ROS1 inhibitor for the treatment of carcinoma, an ERK kinase 1 and 2 inhibitor, a DNA replication inhibitor and a BRAF inhibitor), an endothelin A receptor blocker, two novel factor Xa inhibitors (anticoagulants), a motilin receptor agonist (gastrointestinal), a marketed thyroid hormone, an antiepileptic agent (AMPA antagonist), two novel dipeptidyl-peptidase-4-inhibitors for treatment of type 2 diabetes, a PDE4 inhibitor for treatment of COPD and asthma, a JAK3 inhibitor to treat rheumatoid arthritis, a histamine H1 antagonist, a microsomal triglyceride transfer protein inhibitor, a traded vitamin, a drug to treat electrolyte metabolic disorders, an anticonvulsant clinical candidate (potassium agonist) and two antibacterial drugs (a traded drug inhibiting DNA-dependent RNA polymerase and an investigational peptide deformylase inhibitor).
|
|
Variability of oral bioavailability in humans (inter-subject variability)
|
|
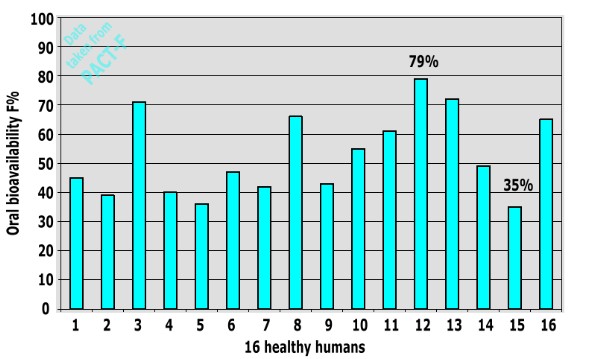
The oral bioavailability of a drug can be different in individual human subjects. As an example, we present results from a clinical trial on a traded antibiotic conducted with 16 healthy human subjects:
|
|
|
|
This example shows that there is substantial inter-subject variability between individual humans: Oral bioavailability values between 35% and 79% were obtained (MAE= 16.64).
In order to estimate inter-subject variability of oral bioavailability, a search in PACT-F was carried out. This yielded several clinical trials, in which the individual oral bioavailability of each subject was specified in the original literature.
We calculated absolute differences between every data point in one clinical trial and averaged them. As a result the average inter-subject difference in oral bioavailability between two humans was estimated (MAE=15-19).
|
|
Species differences in oral bioavailability: the Ciprofloxacin example
|
|
Through the development of the knowledge base PACT-F it was possible for the first time to analyse and quantify differences in oral bioavailability between humans and animals based on a large number of drugs.
A detailed comparison of experimental results from humans and animals showed that the difference between oral bioavailability values from humans and animals was high (MAE=24-27) and only a low to medium correlation (r=0.37-0.56) exists.
Furthermore this comparison identified a large number of previous unknown examples of species differences in drug discovery and development. Here we describe the combined research results of bioavailability studies on Ciprofloxacin in humans and several animals, which has been, to our knowledge, not published before.
Ciprofloxacin is a broad spectrum antibiotic. It is an inhibitor of bacterial topoisomerase, which is required for bacterial DNA replication and therefore reduces the growth of bacteria.
Ciprofloxacin was discovered by Bayer A.G. in 1981 and was the first fluoroquinolone antibiotic, which reached the market. In 1987 the drug was first approved in Europe and in the United States under different trade names (Cipro, Ciprobay, etc.). It is used world-wide and still receives an enormous amount of sales.
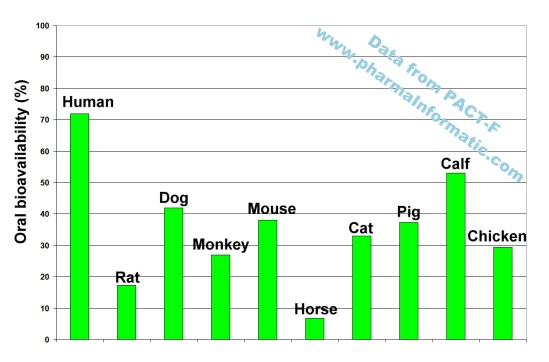
An analysis of all records about Ciprofloxacin in PACT-F showed substantial inter-species differences in oral bioavailability. In humans a high oral bioavailability was found. Interestingly all oral bioavailability results from nine different animal species were less than 55%. The following figure shows the oral bioavailability results measured in ten different species:
|
|
|
|
This example shows that large differences in oral bioavailability can exist between humans and animals. Several alternative methods to animal trials such as quantitative structure bioavailability relationships (QSBR) are available to estimate human oral bioavailability. Since the quality of prediction from those computer-generated models was found to be much more reliable than animal trials, the application of these models will improve the efficiency and cost-effectiveness of clinical trials in humans.
|
|
Further information on oral bioavailability of blockbuster drugs in humans and animals (ARIPIPRAZOLE, OMEPRAZOLE, ESOMEPRAZOLE and DULOXETINE).
|
|
Conclusions
|
|
Oral bioavailability is one of the most important properties in drug discovery and development. A high oral bioavailability reduces the amount of an administered drug necessary to achieve a desired pharmacological effect and therefore could reduce the risk of side-effects and toxicity. A poor oral bioavailability can result in low efficacy and higher inter-individual variability and therefore can lead to unpredictable response.
For the first time, it is now possible to provide a fundamental analysis, if animal trials are useful and reliable to estimate human outcome. Due to the large amount of knowledge within PACT-F, we were able to analyse and quantify these differences in oral bioavailability based on a large number of investigated drugs.
These results can have a large impact on future drug development, on toxic risk assessment of chemicals and on the use of animals as a model, since species differences in oral bioavailability affect a large variety of interdisciplinary biological research fields.
Our main research findings are:
- Animal trials are not reliable to estimate oral bioavailability in humans. Oral bioavailability measured in animals differs substantially from oral bioavailability results in humans. The mean absolute error was high (MAE=24-27) and only a low to medium correlation was obtained between experimental results in humans and animals.
- Drug-uptake studies in animals can hamper drug discovery and development. Aripiprazole and Esomeprazole, the most sold drugs of 2013, have low oral bioavailability in animals, but drug-uptake in humans is high. From an ethical AND a commercial point of view, it is not advisable to carry out animals studies on oral bioavailability and trust in their results.
- Alternative methods were found to be much more reliable than animal trials. Several computer-models such as expert systems or QSBR developed by us and other research groups, estimate human oral bioavailability much more precisely than animal trials. Oral bioavailability predictions of IMPACT-F were as accurate as the common deviation between individual humans taking part in the same clinical trial (MAE=15-19).
It is estimated that more than 100 Million animal experiments are carried out in preclinical research and toxicity testing every year. One should evaluate the benefit of animal trials on oral bioavailability, if the content of information gained is low, costs involved are high, and a reduction of animal trials is always worthwhile.
It has taken years to obtain these results, since an excessive amount of data had to be integrated and compared. Now we have proof that computer-models are significantly more efficient and reliable than animal trials and hopefully they will replace useless animal trials soon.
|
|
Contact:
Dr. Wolfgang Boomgaarden
Founder and CEO of PharmaInformatic
(email)
We will continuously publish novel findings and results from our research on this website.
Follow us on Twitter or connect via LinkedIn:
 
|
|
|
|
REPLACEMENT OF ANIMAL TESTING!
|