LEAD
OPTIMIZATION
with
IMPACT-F:
==> selects oral bioavailable
drugs
==> increases efficacy of clinical trials
==> results
directly available
Only compound structure needed
==> no further assays, synthesis
or costs
|
Collaborations, clients & partnerships: |
||
|
|
||
|
EXITOX-II: Explain Inhalation Toxicity II |
||
|
|
Animal-free mechanism-based toxicity testing – predict toxicity after repeated dose inhalation exposure by using a read across approach. |
|
|
|
||
|
|
||
|
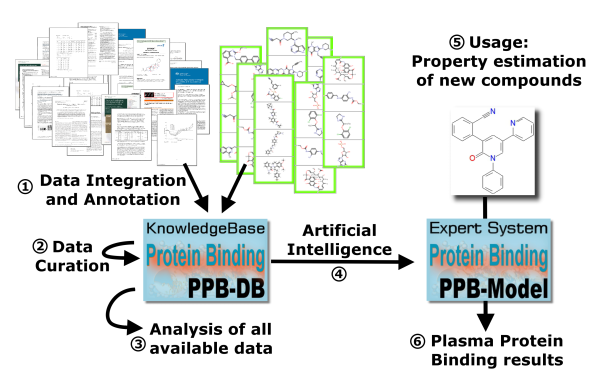
A validation of the method resulted in a high quality of prediction: Based on a large validation dataset a low mean absolute error and a high correlation between experimental and predicted PPB values was observed (prediction of protein binding in human plasma). An outstanding quality of prediction was achieved: Compared to conventional methods which forecast plasma protein binding by compound structure the error of prediction was reduced by half. The project results were presented in January 2020 at the BMBF-Statusseminar in Berlin.
|
||
|
|
|
|
|
|
||
|
Our technology has been used by pharmaceutical companies in many different therapeutic areas such as cancer, inflammation, autoimmune diseases, antivirals for selection and prioritisation of drug candidates, to optimise prodrugs and to evaluate oral bioavailability before advanced clinical trials in humans.
|
||
|
|
||
|
CRACK IT DoCE Challenge (Unilever, Shell) |
||
|
|
DoCE (Dosing for Controlled Exposure): Dosing strategies for characterising in vitro dose-responses with increased relevance for in vivo extrapolation. |
|
|
|
||
|
|
The CRACK IT Challenge is the NC3Rs’ response to the changing environment in the biosciences. The NC3Rs is a UK-based scientific organization dedicated to replacing, refining and reducing the use of animals in research and testing. |
|
|
|
||
|
|
||
|
|
||
|
Collaboration with Avivia BV |
||
|
Avivia BV and PharmaInformatic have entered into a research collaboration agreement. Under the terms of the agreement, PharmaInformatic has estimated human oral bioavailability of prodrugs for the treatment of cancer. We used the Impact-F system to assess the oral bioavailability of two different prodrugs of the same drug that we had in development. We did not have the resources to investigate the oral bioavailability of both with the traditional approach in animals (labelling the mand full pharmacokinetics). The Impact-F analyzed our molecules and it found out that chemically speaking it was very comparable to some other prodrugs and drugs from which oral bioavailability data was available and we used this to make a selection. Hans Platteeuw, Chief Executive Officer at Avivia BV
|
||
|
|
||
|
PharmaInformatic and UNIZYME Laboratories A/S have entered into a research collaboration for the development of new therapeutic agents for the treatment of inflammatory diseases. The research collaboration is initiated and supported by the European Union IN2LifeSciences project and by the Interreg IVB North-West Europe programme. PharmaInformatic uses the expert system IMPACT-F to prioritise dipeptidyl peptidase I inhibitors (DPPI, cathepsin C) based on their estimated oral bioavailability in humans. |
||
|
|
||
|
PharmaInformatic and BRIDGE BIORESEARCH PLC have signed a collaboration agreement. Under this agreement PharmaInformatic applies the expert system IMPACT-F in order to predict the oral bioavailability of drug candidates for the treatment of type 2 diabetes. |
||
|
Søren Stenderup explains the collaboration in the newsletter from BioPeople, Denmark: Søren Stenderup, CEO at Bridge Bioresearch Diabetes mellitus type 2 is a metabolic disorder which affects a large number of people. According to the World Health Organisation, about 347 million people suffer from diabetes worldwide. About 90% of them suffer from diabetes type 2. |
||
|
The calculation is based on reliable computational models, which have been derived from the largest knowledge base on bioavailability worldwide (PACT-F). |
||
|
Several research collaborations are supported by the European Union IN2LifeSciences project and by the Interreg IVB North-West Europe programme. IN2LifeSciences supports SME with the development or commercialisation of their innovations. PharmaInformatic is a technology provider within the IN2LifeScience program. |
||
|
|
||
|
|
||
|
|
||
|
Looking for similar drug structures and bioavailability data related to your research project? |
||
|
|
||


|
© Copyright 2004-2021 PharmaInformatic Boomgaarden. All rights reserved. Site map Contact Terms of Use Imprint |
Evaluate
efficiency of drugs
before
advanced clinical trials:
==>
IMPACT-F
BIOAVAILABILITY
ESTIMATION
(more)