Detecting new antiviral drugs
MolScores-Antivirals detects future antiviral drugs. The expert system has been validated with novel antiviral substances, which are now in clinical development.

An expert system to identify suitable
antiviral candidates.
New Chemical Entities (NCE)
are recognised by MolScore- Antivirals.

An expert system for finding
suitable
drug candidates.

An expert system to identify suitable antibacterial candidates.
An expert system to calculate oral bioavailability in humans.
|
NCE |
Company |
Country |
|
Japan |
||
|
USA |
||
|
USA / France |
||
|
Panacos Pharmaceuticals Inc. |
USA |
|
|
Japan |
||
|
USA |
||
|
ZymeTx, Inc. |
USA |
|
|
ZymeTx, Inc. |
USA |
|
|
BioAlliance Pharma |
France |
|
|
USA |
||
|
Sweden / United Kingdom |
||
|
Trimeris Inc. |
USA |
|
|
Tibotec Pharmaceuticals Ltd. |
Belgium / Ireland / USA |
|
|
USA |
||
|
Ambrilia Biopharma Inc. |
Canada |
|
|
USA |
||
|
Migenix Inc. |
Canada |
|
|
USA |
||
|
Japan |
Valtorcitabine is a drug candidate for the treatment of hepatitis B virus (HBV) and is currently in combination with telbivudine in a phase IIb clinical program which was initiated in 2004. In preclinical studies valtorcitabine demonstrated profound and highly specific antiviral activity against HBV.
SM-322377 has potent antiviral activity against HIV-1, see Mimoto T, Terashima K, Nojima S, Takaku H, Nakayama M, Shintani M, Yamaoka T, Hayashi H. Structure-activity and structure-metabolism relationships of HIV protease inhibitors containing the 3-hydroxy-2-methylbenzoyl-allophenylnorstatine structure. Bioorg Med Chem. 2004 Jan 2;12(1):281-93.
Valtorcitabine:
The Food and Drug Administration (FDA) announced in June 2003, the approval of atazanavir sulfate, a protease inhibitor to be used in combination with other anti-retroviral agents for the treatment of patients with HIV infection. Atazanavir (Reyataz®) is manufactured by Bristol-Myers Squibb.
FZ-41 is a lead candidate which has been selected for development. Phase 1 trial are expected to initiate in 2007. Further information: Mousnier A, Leh H, Mouscadet JF, Dargemont C. Nuclear import of HIV-1 integrase is inhibited in vitro by styrylquinoline derivatives. Mol Pharmacol. 2004 Oct;66(4):783-8. Medline Abstract.
FZ-41:
Atazanavir (BMS-232632):
Elvucitabine is an L-cytosine nucleoside analogue which is in phase II clinical trials. Research indicates that its antiviral activity results from inhibition of the HIV reverse transcriptase enzyme.
Valomaciclovir is a broad-spectrum anti-herpes agent, which targets varicella-zoster DNA-polymerase. A clinical phase II trial is completed.
Valomaciclovir (RP-606):
Elvucitabine (ACH-126,443 or Beta-L-Fd4C):
Celgosivir is a novel, oral antiviral agent under development for the treatment of chronic hepatitis C virus (HCV) infection. Migenix Inc. obtained Health Canada CTA approval to initiate a Phase IIa study in HCV patients in September 2004.
Maribavir is an antiviral compound in development by ViroPharma. It is a orally bioavailable antiviral drug with a unique mechanism of action against cytomegalovirus. It is a potent member of a new class of drugs called benzimidazole ribosides. Maribavir inhibits viral DNA assembly. ViroPharma announced in November 2005 that it had completed enrollment in its Phase 2 clinical study.
Maribavir:
Celgosivir (MX-3253):
T20 (FUZEON®) was approved by the US FDA and European Commission in 2003. It was the first fusion inhibitor.
Tibotec Pharmaceuticals Ltd. announced in March 2006 that the New Drug Application (NDA) for TMC-114, an investigational HIV protease inhibitor, has been accepted for priority review by the United States Food and Drug Administration (FDA). Phase III clinical trials of TMC-114 are currently ongoing in HIV-1 infected patients.
TMC-114:
T-20 (DP-178):
The parainfluenza virus inhibitor BCX 2855 was designed based on the three-dimensional structure of the hemagglutinin-neuraminidase protein, for further information: Alymova IV, Taylor G, Takimoto T, Lin TH, Chand P, Babu YS, Li C, Xiong X, Portner A. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob Agents Chemother. 2004 May;48(5):1495-502.
Peramivir was designed to treat and prevent various types of flu and may have utility against strains including influenza A and B, avian influenza (“bird flu”). Peramivir is part of a new class of antiviral agents that work by inhibiting viral neuraminidase, an enzyme essential for the influenza virus to replicate and infect its hosts. In laboratory tests, peramivir has been shown to be a potent and selective inhibitor of influenza A and B neuraminidases.
In August 2009 Peramivir has entered a phase III clinical trial for the treatment of patients who are hospitalized due to serious influenza.
Peramivir (RWJ-270201, BCX-1812):
BCX-2855:

An expert system for finding molecules with antiviral activity.
Our
expert systems
are based on novel models and innovative methods in the field of drug design.
We use proprietary molecular descriptors and a huge amount of data and knowledge from literature to find relationships between molecular structures and biological activity.
The knowledge of our expert systems is used by our clients to filter large compound libraries.
Please be aware that drug status changes and the information provided here may be outdated.
For actual and comprehensive information about current drug status or clinical trials please check on the company’s home page.
Panacos Pharmaceuticals announced the commencement of a multicenter Phase 2b trial studying the effects of combination therapy in HIV-infected patients on June 2006. In August 2009 Peramivir has entered a phase III clinical trial for the treatment of patients who are hospitalized due to serious influenza.
Bevirimat:
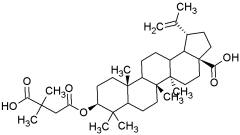
Bevirimat (PA-457) is the first in a new class of HIV drugs called maturation inhibitors. It blocks HIV maturation by inhibiting the final step in the processing of the HIV Gag protein. The resulting virus particles are incapable of spreading infection around the body.
92% of clinical candidates have been correctly classified as antiviral molecules, which are suitable for further development into drugs.
53% of these molecules had a prediction result between 0,9 and 1,0. This validation demonstrates the excellent prediction capability of MolScore-Antivirals to detect antiviral clinical candidates.
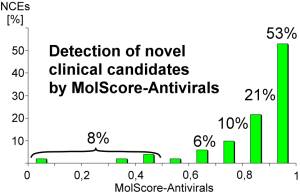
Below are some examples of substances which are currently in development by various companies. New chemical entities can be recognised by MolScore-Antivirals, regardless of substance-classes, mechanism of action or molecular weight.
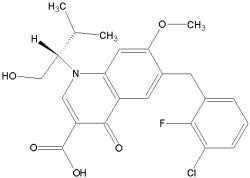
JTK-303/GS-9137 is a highly selective HIV-1 integrase inhibitor with potent antiviral activity against HIV-1. It was discovered through screening of inhibitory activity against recombinant HIV-1 integrase at the Central Pharmaceutical Research Institute of Japan Tobacco Inc.
The compound has potential as a novel, orally bioavailable anti-HIV agent.
In July 2008 Gilead initiated a Phase III clinical trial.
JTK-303/GS-9137:
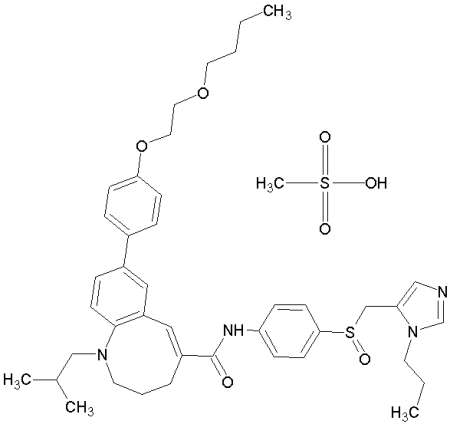
TAK-652 is a promising candidate as a novel entry inhibitor of HIV-1 and was safe and well tolerated in humans (clinical trial, phase I), see Baba M, Takashima K, Miyake H, Kanzaki N, Teshima K, Wang X, Shiraishi M, Iizawa Y. TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favourable pharmacokinetics in humans. Antimicrob Agents Chemother. 2005 Nov;49(11):4584-91.
TAK-652:
CJ 4-16-4 is a potent inhibitor of respiratory syncytial virus (RSV). The antiviral activity was specific; influenza and herpes simplex viruses were not inhibited.
CJ 4-16-4:
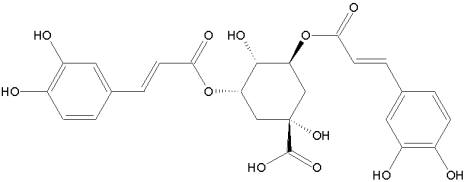
ZX-2401 is a broad-spectrum inhibitor of viruses in the flaviviridae family. The compound inhibited yellow fever virus, dengue virus, bovine viral diarrhea virus, banzi virus and West Nile virus. The mechanism of action is currently unknown. ZX-2401 is also a nucleoside analog and it could have similar mechanism of action like ribavirin.
ZX-2401:
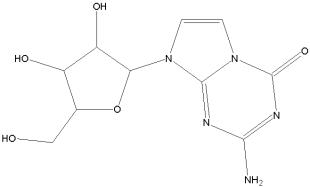
For further information, see Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D, Ingravallo P, Jiang C, Kong R, Lu J, Pichardo J, Prongay A, Skelton A, Tong X, Venkatraman S, Xia E, Girijavallabhan V, Njoroge FG. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob Agents Chemother. 2006 Mar;50(3):1013-20.
In January 2006 Schering-Plough reported that the U.S. FDA has granted Fast Track designation to its investigational oral hepatitis C protease inhibitor SCH 503034, which is currently in Phase II clinical development for the treatment of chronic hepatitis C virus infection.
SCH 503034 is a HCV NS3 protease inhibitor and was generated using structure-assisted design.
It has demonstrated efficacy a HCV replicon system with virtually no cellular toxicity and has been advanced for clinical evaluation.
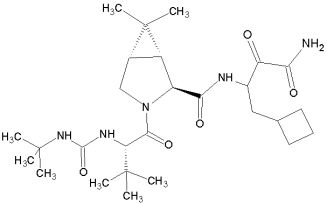
Ambrilia’s PPL-100 is a promising protease inhibitor which binds specifically to HIV-1 protease. The compound is currently in phase I human clinical trial (June 2006). PPL-100 is the phosphorylated prodrug of PL-100.
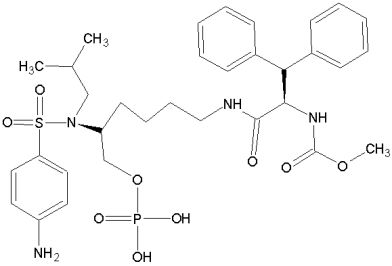
Our
expert systems
are based on novel models and innovative methods in the field of drug design. Further Links:
PACT-F
Drug discovery
Drug development
Cheminformatics
ADME
Pharmacokinetics
Bioavailability
Computational chemistry
Molecular
modeling
Clinical trials
Preclinical
Research
Our
expert systems
are based on novel models and innovative methods in the field of drug design.
We use proprietary molecular descriptors and a huge amount of data and knowledge from literature to find relationships between molecular structures and biological activity.
Further Links:
PACT-F
Drug design
Drug discovery
Drug development
Cheminformatics
ADME
Pharmacokinetics
Bioavailability
Computational chemistry
Molecular
modeling
Clinical trials
Preclinical
Research
Please be aware that drug status changes and the information provided here may be outdated.
For actual and comprehensive information about current drug status or clinical trials please check on the company’s home page.

An expert system for finding molecules with antiviral activity.
Our
expert systems
are based on novel models and innovative methods in the field of drug design.
We use proprietary molecular descriptors and a huge amount of data and knowledge from literature to find relationships between molecular structures and biological activity.
The knowledge of our expert systems is used by our clients to filter large compound libraries.
Please be aware that drug status changes and the information provided here may be outdated.
For actual and comprehensive information about current drug status or clinical trials please check on the company’s home page.


|
© Copyright 2004-2021 PharmaInformatic Boomgaarden. All rights reserved. Site map Contact Terms of Use Imprint |


